Pipeline
Advancing Obexelimab and Orelabrutinib for Patients with Autoimmune Diseases
Zenas is committed to being a leader in the development and commercialization of transformative therapies for patients with autoimmune diseases. We are advancing a balanced portfolio of complementary mechanisms and modalities with best-in-class blockbuster potential across multiple therapeutic areas. This begins with obexelimab, a potential I&I franchise within a molecule, and orelabrutinib, a potential progressive multiple sclerosis franchise within a molecule.

Obexelimab
-
Immunoglobin G4-related Disease (IgG4-RD)
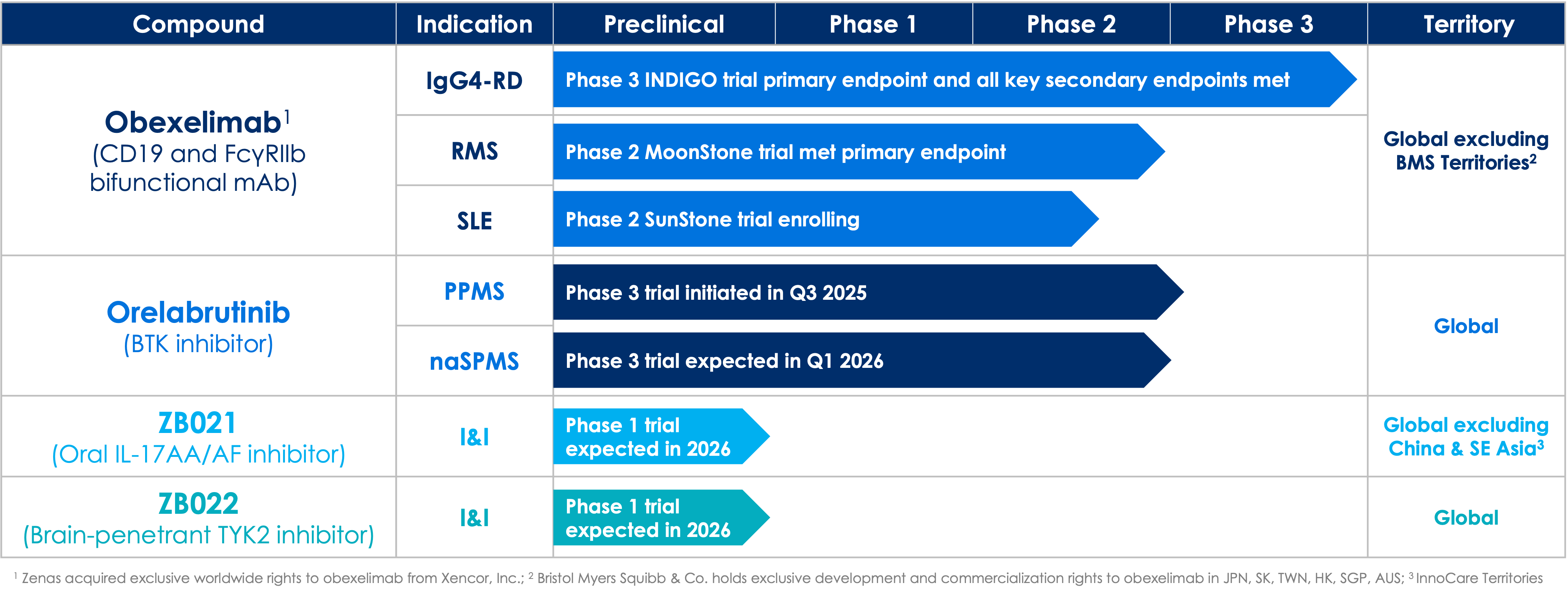
Phase 3 INDIGO trial met primary and all key secondary endpoints. Zenas anticipates submitting a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) for obexelimab in IgG4-RD in the second quarter of 2026 and a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) in the second half of 2026. Learn more at: NCT05662241.
-
Relapsing Multiple Sclerosis (RMS)
In the Phase 2 MoonStone trial, obexelimab met the primary endpoint, demonstrating a highly statistically significant 95% relative reduction in the cumulative number of new gadolinium (Gd)-enhancing (GdE) T1 hyperintense lesions over week 8 and week 12 compared with placebo (p=0.0009). The trial enrolled 116 patients and is being conducted at 47 sites in 12 countries. Zenas plan to report 24-week MoonStone trial results in the first quarter of 2026. Learn more at: NCT06564311.
-
Systemic Lupus Erythematosus (SLE)
We are enrolling the SunStone trial, a Phase 2 global, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of obexelimab when used to reduce disease activity in patients with SLE. We expect to enroll approximately 190 patients and conduct the trial at multiple sites worldwide. Patients will be randomized 1:1 to obexelimab or placebo over 24 weeks. The primary endpoint will be the percentage of responders, defined by BILAG-based Composite Lupus Assessment (BICLA), with a reduction of SLE disease activity at week 24. Learn more at: NCT06559163.
Orelabrutinib
-
Primary Progressive Multiple Sclerosis (PPMS)
We are advancing a pivotal, global, Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial evaluating orelabrutinib in patients with PPMS. The trial will evaluate the safety and efficacy of orelabrutinib 80 mg once daily (QD) dosing compared to placebo, with a primary endpoint of time to onset of 12-week composite confirmed disability progression (cCDP). Learn more at: NCT07067463.
-
Non-Active Secondary Progressive Multiple Sclerosis (naSPMS)
We expect to initiate a pivotal, global, Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical trial evaluating orelabrutinib in patients naSPMS in the first quarter of 2026. The trial will evaluate the safety and efficacy of orelabrutinib 80 mg QD dosing compared to placebo, with a primary endpoint of time to onset of 24-week CDP.
Pipeline
-
Other Pipeline Programs
Beyond our lead product candidates, obexelimab and orelabrutinib, we are advancing a pipeline of clinical programs for the potential treatment of other autoimmune diseases that we may continue to develop and ultimately commercialize on our own or with partners. These pipeline programs include ZB021, ZB022, ZB002 and ZB004.